Mitophagy represents one of the most fascinating and clinically relevant cellular quality control mechanisms in our understanding of aging and longevity. This selective form of autophagy specifically targets damaged or dysfunctional mitochondria for elimination, serving as the cell’s sophisticated recycling system that maintains mitochondrial health and energy production capacity. Emerging research demonstrates that mitophagy dysfunction underlies many age-related diseases, while targeted interventions to enhance mitophagy show remarkable promise for extending healthspan and promoting cellular resilience.
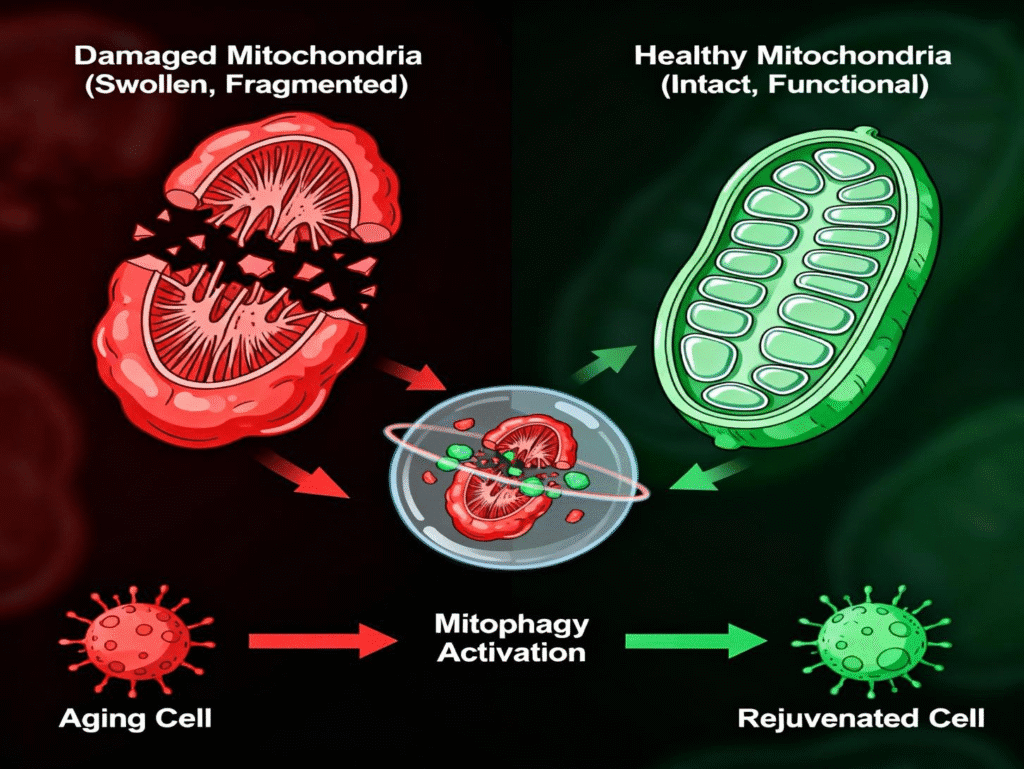
Illustration of the mitophagy process showing selective autophagy of damaged mitochondria.
Understanding Mitophagy: The Cellular Quality Control System
Mitophagy, derived from “mitochondria” and “phagy” (eating), is a highly regulated process by which cells selectively identify, isolate, and degrade damaged or excess mitochondria. Unlike general autophagy, which indiscriminately removes cellular components, mitophagy employs sophisticated molecular machinery to specifically target mitochondria that have lost their functional integrity or are no longer needed.
The process begins when mitochondria experience stress, damage, or functional decline. These compromised organelles undergo membrane depolarization, a critical signal that triggers the mitophagy cascade. The cell’s surveillance systems detect this change and initiate a complex molecular program designed to remove the defective mitochondria while preserving healthy ones safely.
The PINK1/Parkin Pathway: Molecular Guardians of Mitochondrial Health
The most extensively studied mitophagy pathway involves two key cellular proteins that work together like a sophisticated security system: PINK1 and Parkin. Think of PINK1 as a quality inspector that typically enters healthy mitochondria and gets quickly recycled when everything is working correctly. However, when a mitochondrion becomes damaged or starts to malfunction, it loses its electrical charge (like a battery running down), and PINK1 can no longer enter correctly. Instead, PINK1 builds up on the outside surface of the damaged mitochondrion, where it acts like a bright red flag that signals to the cell: this mitochondrion is broken and needs to be removed, serving as a “eat me” signal.
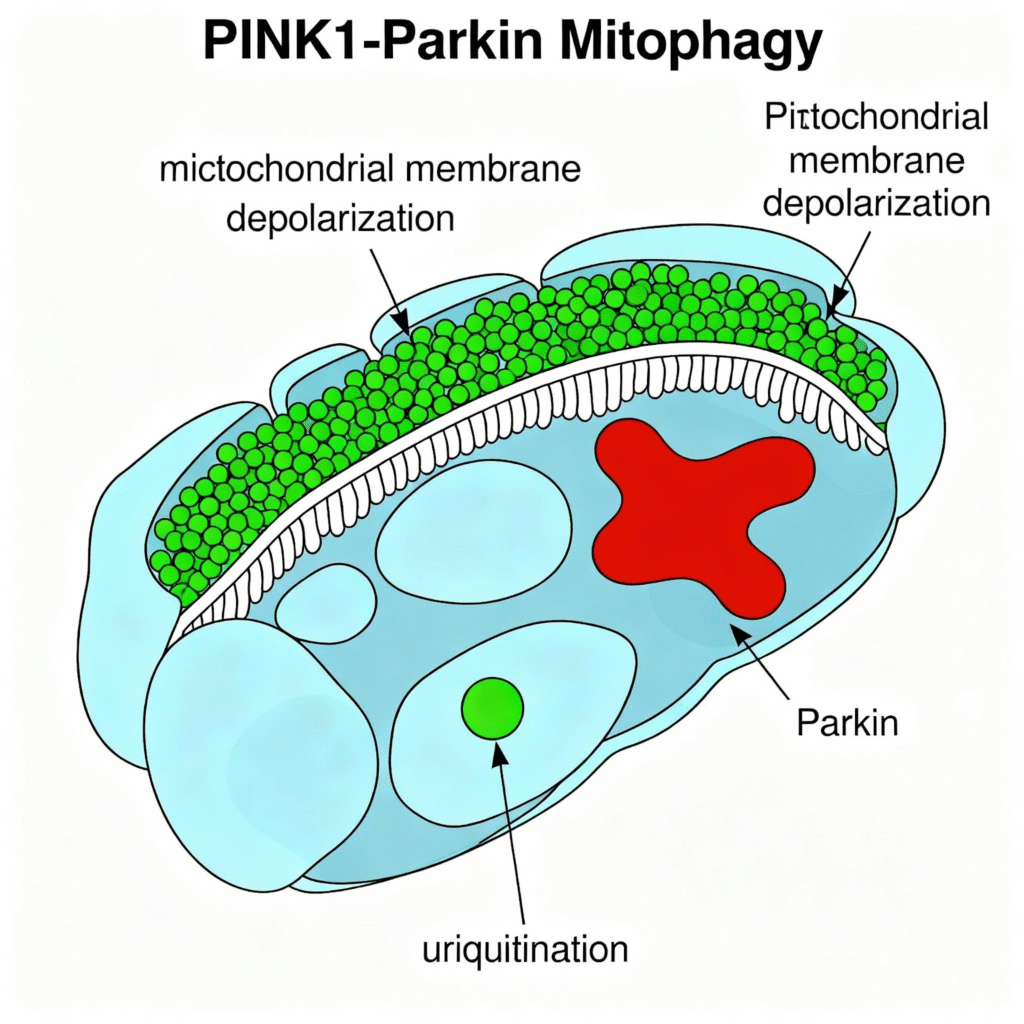
PINK1/Parkin pathway in mitophagy showing molecular mechanisms of damaged mitochondria clearance.
When PINK1 accumulates on the surface of the damaged mitochondrion, it recruits its partner, Parkin, to assist in completing the removal process. Parkin acts like a molecular tagger, attaching special molecular ‘sticky notes’ (called ubiquitin tags) to proteins all over the damaged mitochondrion’s surface. These sticky notes serve as clear instructions that say, ‘remove this immediately.’ Cellular cleanup crews, including a protein called p62, recognize these tags and guide the damaged mitochondrion into a specialized disposal bag called an autophagosome. This bag then carries the tagged mitochondrion to the cell’s recycling center (called lysosomes), where powerful enzymes break it down entirely and recycle its valuable components.”
Alternative Mitophagy Pathways: Receptor-Mediated Clearance
Beyond the PINK1/Parkin security system, cells have developed several other specialized removal services that can directly connect damaged mitochondria to the cellular cleanup machinery. These alternative systems use different molecular workers—proteins with names like NIX, FUNDC1, and cardiolipin—that act as specialized disposal coordinators. Each coordinator is designed to handle mitochondria removal under specific circumstances, such as when cells experience low oxygen levels, during normal development processes, or when the cell is under metabolic stress.
For example, FUNDC1 specializes in removing mitochondria when oxygen levels drop dangerously low, while NIX has a unique job during the development of red blood cells. As red blood cells mature, they must completely eliminate all their mitochondria to make room for oxygen-carrying hemoglobin; NIX ensures this cellular housekeeping is done correctly. Having these diverse removal pathways is like having different specialized cleaning services available: the cell can always call the right service for the right situation, ensuring that mitochondria are removed efficiently, regardless of the challenges the cell faces.
The NAD+/Sirtuin Axis: Orchestrating Mitochondrial Quality Control
A central regulatory hub for mitophagy involves the NAD+/sirtuin signaling network, which has profound implications for aging and longevity. NAD+ (nicotinamide adenine dinucleotide) serves as a critical cofactor for sirtuins, a family of NAD+-dependent deacetylases that regulate numerous cellular processes, including mitophagy.
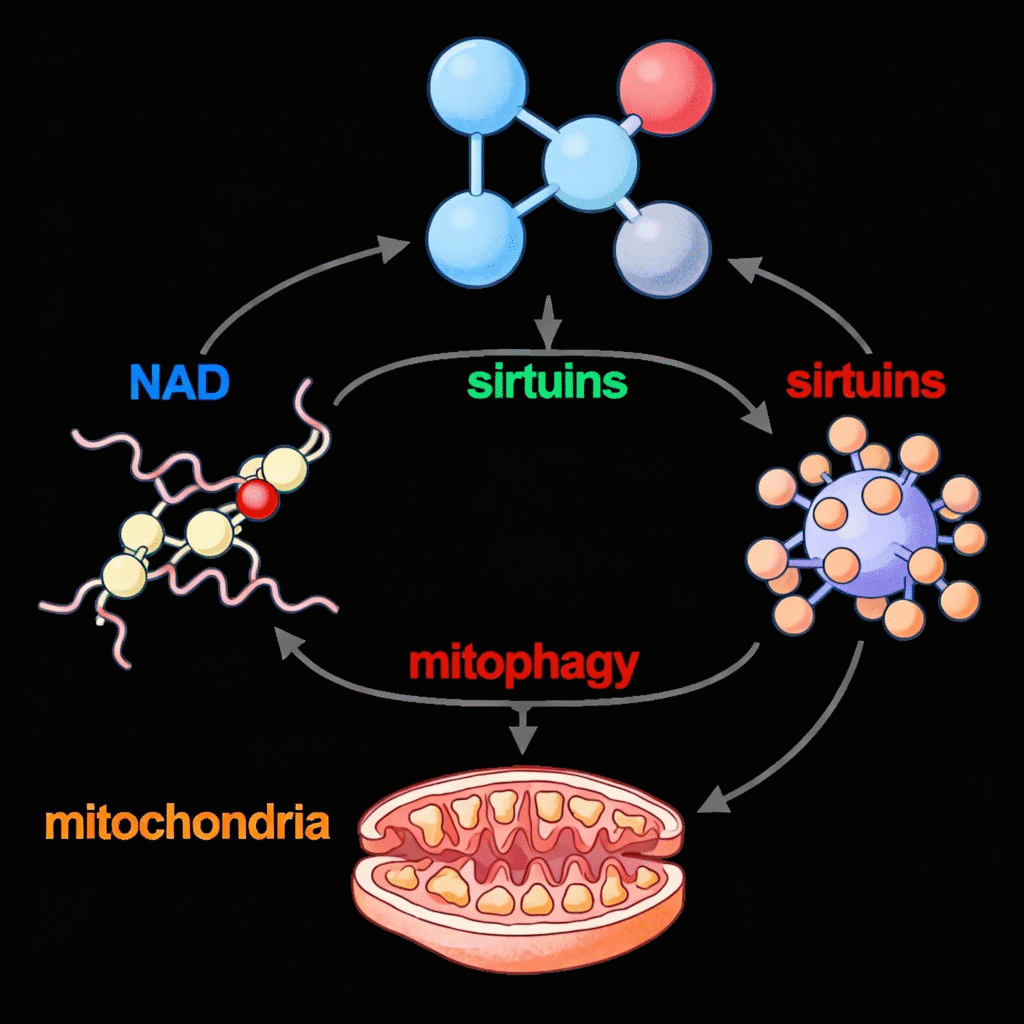
NAD+/Sirtuin signaling pathway regulation of mitophagy and mitochondrial quality control.
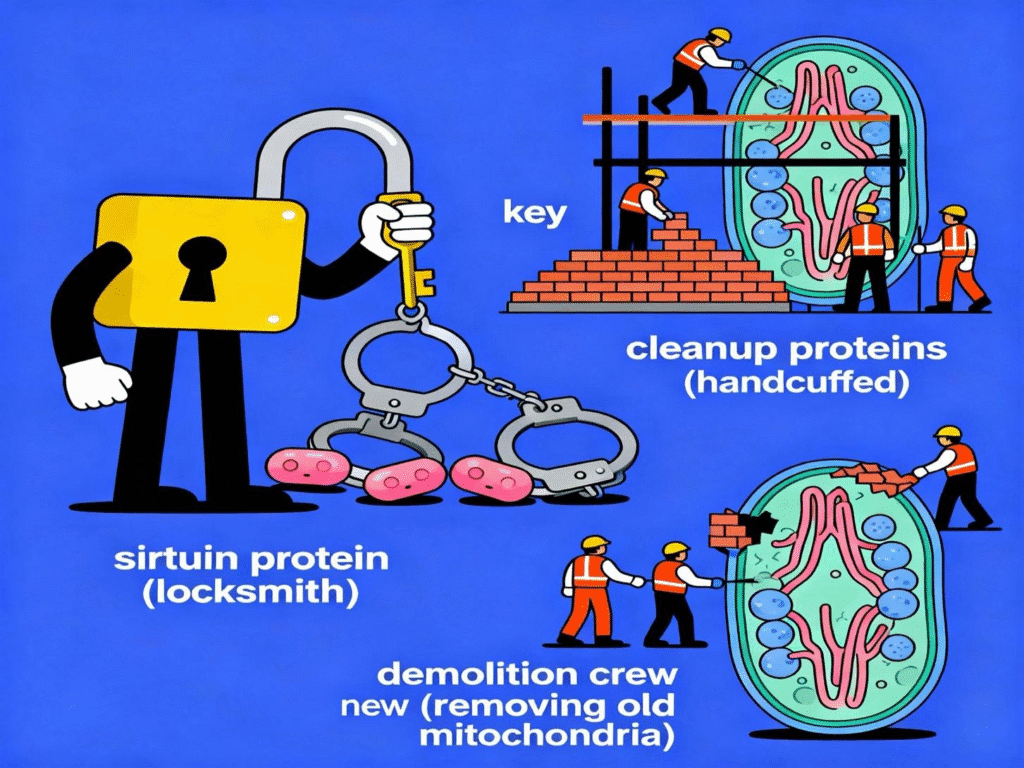
SIRT1, SIRT3, and other sirtuins function as cellular renovation managers, promoting mitophagy through various mechanisms. They do this by removing molecular ‘brakes’ from key cleanup proteins through a process called deacetylation—imagine proteins wearing small molecular handcuffs called acetyl groups that prevent them from working correctly. The sirtuins act like skilled locksmiths, removing these acetyl group handcuffs so the cleanup proteins can move freely and do their jobs effectively. Sirtuins also direct the construction of brand-new mitochondria. This creates a beautifully coordinated system that works like a home renovation crew: while the demolition team removes old, damaged mitochondria, the construction team simultaneously builds fresh, healthy replacements. This tag-team approach ensures that the cell’s energy production never gets interrupted.
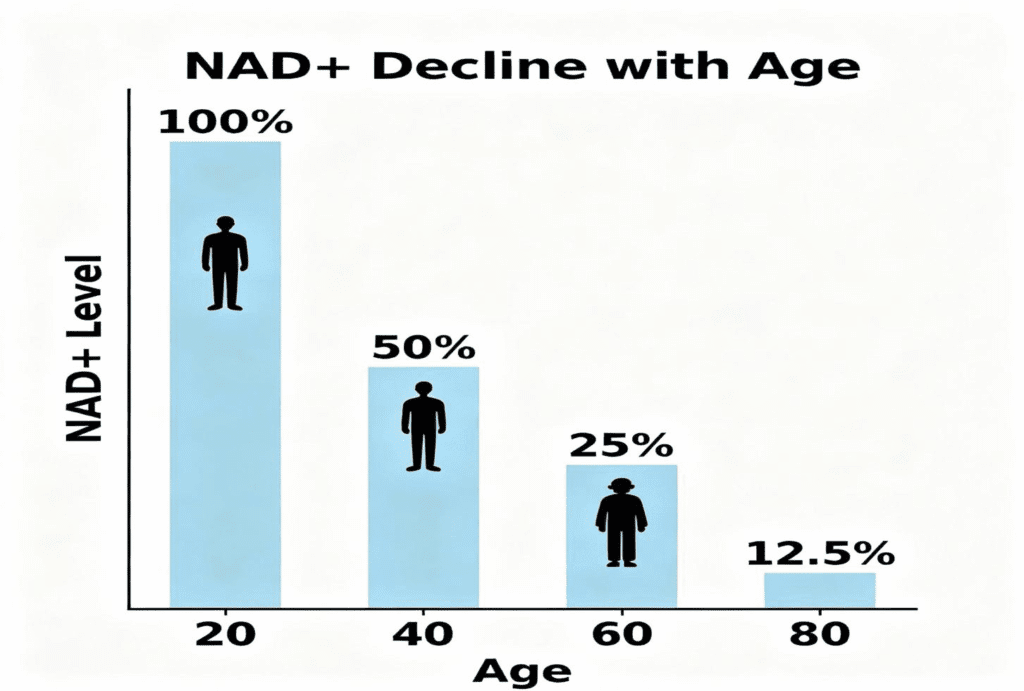
Research shows that as we age, our NAD+ levels—the essential fuel that powers these sirtuin-regulating enzymes—drop significantly. When NAD+ levels are low, the sirtuins cannot perform their functions effectively as molecular locksmiths, resulting in impaired mitophagy efficiency and a potentially hazardous accumulation of damaged mitochondria that should have been removed. This age-related decline in the NAD+/sirtuin system appears to be one of the fundamental reasons why our cells age and our metabolism becomes less efficient over time. The following illustration demonstrates these concepts.
NAD+ levels decline significantly with age, coinciding with a reduction
in mitophagy efficiency and the accumulation of damaged mitochondria. This age-related decline in the NAD+/sirtuin axis appears to be a fundamental driver of cellular aging and metabolic dysfunction. Unless some types of interventions are undertaken, the consequences of NAD decline can be disastrous. Remember, decreased NAD results in decreased mitochondrial function, which leads to aging.
Mitophagy and Age-Related Disease
When the cellular cleanup system breaks down, it creates a cascade of serious health problems, particularly affecting our brains and hearts—two organs that demand enormous amounts of energy to function correctly due to the large number of mitochondria they possess. Think of what happens when garbage collection stops in a city: waste piles up everywhere, creating toxic conditions that make normal life impossible.
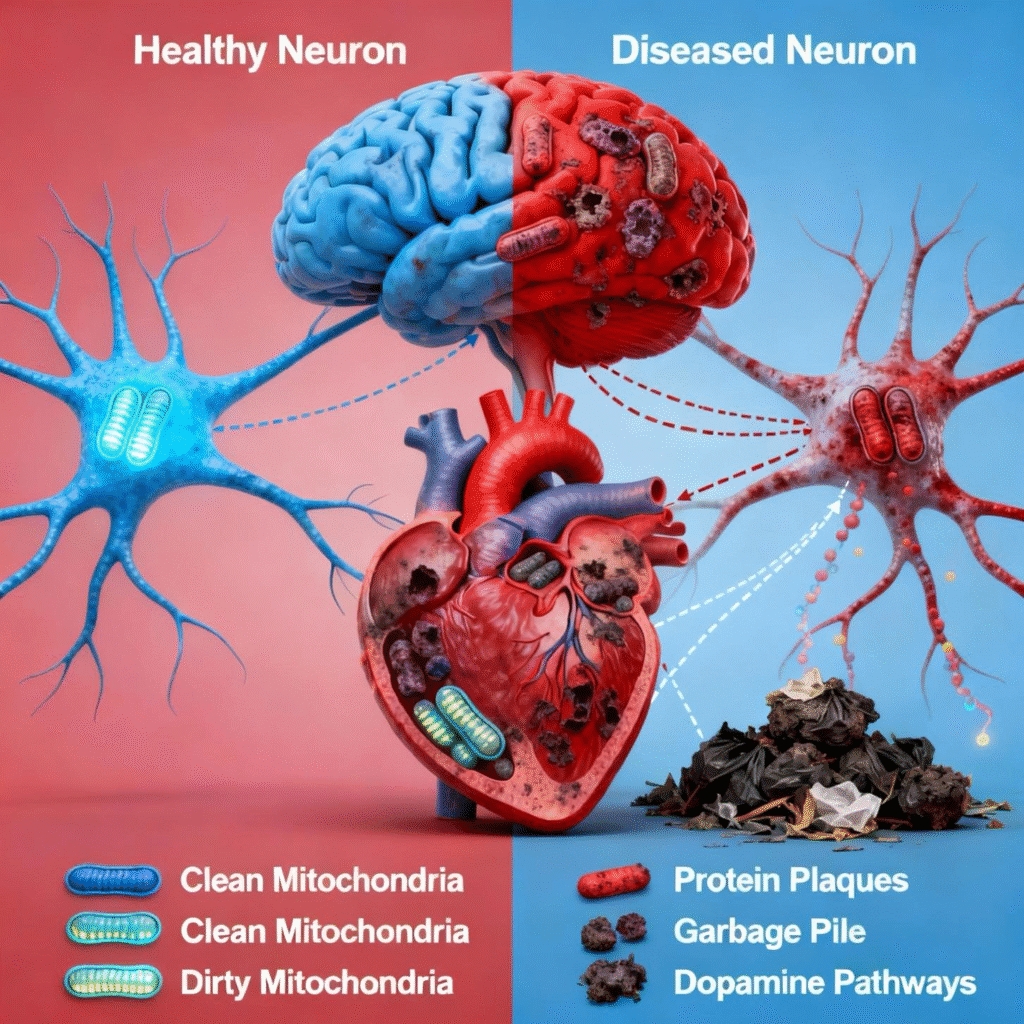
In Parkinson’s disease, genetic mutations damage the PINK1 and Parkin cleanup crews we discussed earlier, leading to a complete breakdown in mitochondrial maintenance within the brain cells that produce dopamine—the chemical messenger responsible for controlling movement. Without proper cleanup, these crucial brain cells become clogged with damaged mitochondria and eventually die, leading to the characteristic tremors and movement problems.
Alzheimer’s disease follows a similar pattern of cellular neglect. When mitophagy fails in brain neurons, the cells can’t generate enough energy to function normally, and toxic waste products, such as amyloid plaques and tau proteins, begin accumulating like sludge in a clogged drain. This creates a vicious cycle where energy-starved brain cells become increasingly unable to clear the very toxins that are poisoning them.
Your heart faces equally serious consequences when mitophagy declines. Think of your heart as a high-performance engine that beats over 100,000 times per day—it absolutely depends on clean, efficient mitochondrial ‘fuel’ to maintain this incredible workload. When the cellular cleanup system fails in heart muscle cells, damaged mitochondria accumulate like dirty oil in an engine, creating harmful free radicals and ultimately causing the heart to weaken and lose its pumping power.
Clinical Interventions: Harnessing Mitophagy for Healthy Aging
The following diagram illustrates the various modalities available at the Pur-Form clinic, which can be instrumental in encouraging mitophagy. Some of these are different compounds, while others are different modalities.
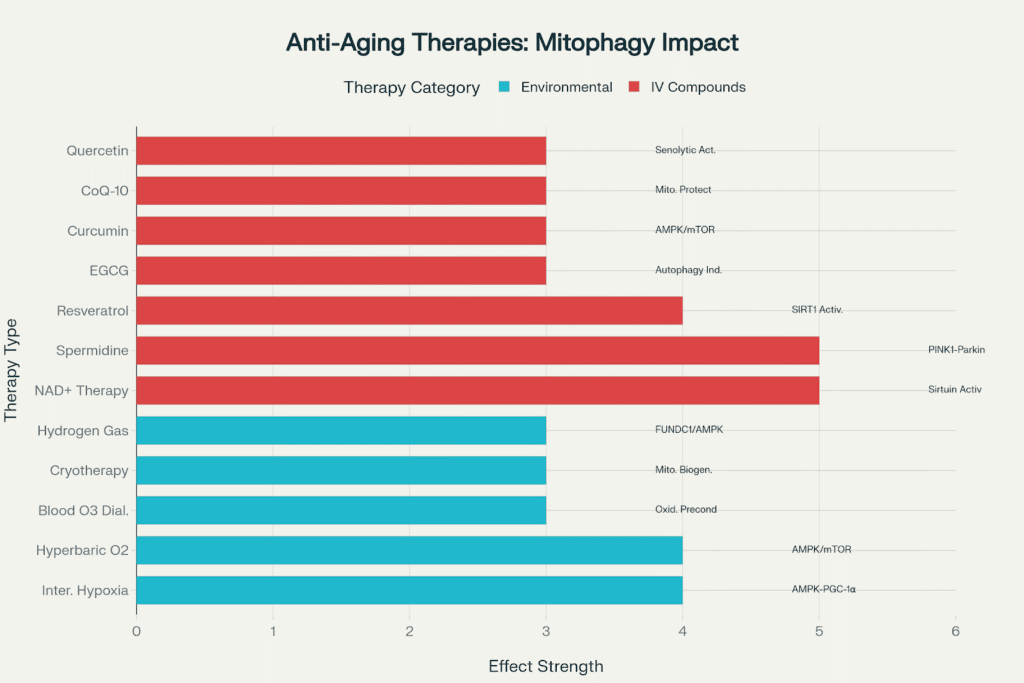
Therapeutic modalities offered at the clinic and their effects on mitophagy pathways.
Environmental Modalities
Intermittent Hypoxia Therapy works like high-altitude training for your cells, emerging as one of the most powerful ways to supercharge your cellular cleanup system. Just as athletes train at high altitudes where oxygen is scarce to build stronger cardiovascular systems, this therapy deliberately exposes your cells to controlled periods of slightly reduced oxygen levels, triggering a remarkable cellular fitness response. When your cells experience these brief oxygen challenges, they activate what we can think of as their ’emergency response team’—a sophisticated signaling network involving proteins called AMPK, PGC-1α, and SIRT3. This cellular alarm system doesn’t just panic; instead, it launches a comprehensive renovation program that’s like having a construction crew and demolition team work simultaneously in perfect coordination.
Research shows that this controlled cellular ‘stress training’ accomplishes three remarkable things at once: it repairs existing mitochondrial damage at the microscopic level, activates the cleanup crews to remove worn-out mitochondria more efficiently, and—most impressively—stimulates your cells to build brand new, more efficient mitochondrial powerhouses. It’s like having your cellular engine rebuilt while you’re still driving, ensuring you end up with more power than when you started. This dual-action approach makes intermittent hypoxia therapy particularly effective: while your cells are busy removing cellular waste, they’re simultaneously constructing state-of-the-art replacements, ensuring your energy production never misses a beat.
Hyperbaric Oxygen Therapy (HBOT) works like putting your cells in a high-performance charging chamber, where they’re bathed in pure oxygen under increased pressure—similar to what deep-sea divers experience, but used therapeutically. This oxygen-rich, pressurized environment acts like a master switch, flipping your cellular cleanup system into overdrive through what scientists call the AMPK/mTOR pathway—think of it as your cell’s central command center that controls when to clean house and when to build new structures.
Studies show that this pressurized oxygen treatment dramatically boosts the activity of your cellular janitors. Scientists can actually measure this by looking at specific cleanup indicators: Beclin-1 and LC3 levels increase (similar to more cleaning crews showing up for work), while p62 levels decrease (indicating that cellular garbage is being removed faster than it accumulates). It’s like watching a sluggish cleaning operation suddenly transform into a well-oiled efficiency machine.
HBOT proves particularly powerful in protecting your cells during what doctors call ‘ischemia-reperfusion injury’—essentially what happens when blood flow gets cut off to an area (like during a heart attack or stroke) and then suddenly restored. This situation is like turning off the power to a factory and then suddenly flipping it back on—it can cause dangerous electrical surges. HBOT helps maintain the integrity of your cellular powerhouses during these critical moments and ensures that any mitochondria damaged during the crisis get quickly identified and removed, preventing them from becoming toxic to surrounding healthy cells.
Blood Ozone Dialysis (EBO2) works on a fascinating principle borrowed from nature: the idea that a small, controlled challenge can make you stronger. Think of it like exercise for your cells—just as lifting weights creates tiny muscle tears that heal back stronger, this therapy uses carefully controlled amounts of ozone to create a mild, beneficial stress that ultimately makes your cells more resilient and efficient.
During the treatment, some of your blood is temporarily removed from your body, mixed with precise amounts of medical-grade ozone, and then returned to your circulation. This process is called ‘oxidative preconditioning’—essentially giving your cells a controlled workout that activates their natural defense systems. It’s similar to how people who live at high altitudes develop stronger cardiovascular systems, or how controlled sun exposure helps your skin build protective melanin.
This controlled cellular challenge triggers a remarkable chain reaction throughout your body. Your cells respond by dramatically boosting their production of natural antioxidants—think of these as your body’s internal bodyguards that protect against harmful free radicals. At the same time, the therapy supercharges your mitochondrial quality control systems, meaning your cellular cleanup crews become more efficient at identifying and removing damaged components before they can cause problems.
What makes this therapy particularly elegant is its preconditioning effect. Just as a vaccine prepares your immune system to fight off future infections, ozone preconditioning prepares your mitochondria to handle better the oxidative challenges life presents—whether from pollution, stress, aging, or disease. Your cells essentially develop a stronger, more robust defense system that maintains optimal energy production even under adverse conditions. This cellular resilience training helps explain why patients often report sustained improvements in energy and vitality long after their treatment series is complete.
Cryotherapy and Cold Exposure work like a powerful wake-up call for your cellular renovation crews, triggering what scientists call ‘hormesis’—the beneficial stress response that makes you stronger. Just as jumping into a cold lake initially shocks your system but leaves you feeling energized and alert, cold therapy jolts your cells into a highly productive state where they simultaneously demolish old, worn-out mitochondria while rapidly constructing new, more efficient replacements.
When your body experiences controlled cold stress, it dramatically increases the production of cellular construction managers—proteins with names like PGC-1α and NRF-1. Think of these as experienced foremen who know exactly how to coordinate a significant renovation project. These molecular managers don’t just oversee the cleanup of damaged mitochondria; they also direct the construction of brand new mitochondrial powerhouses, ensuring that your cells end up with a net gain in energy-producing capacity.
What makes cold therapy particularly compelling is that it creates a rapid ‘mitochondrial turnover’—essentially a complete cellular renovation where old, inefficient mitochondria are quickly identified, removed, and replaced with state-of-the-art models. It’s like having a construction crew that can simultaneously tear down an old building while erecting a modern, energy-efficient replacement on the same lot.
The real magic happens when you combine cold exposure with exercise. These two stressors work together like a perfectly coordinated team, creating what scientists call ‘synergistic effects.’ Exercise creates the demand for more energy, while cold exposure provides the cellular wake-up call needed to meet that demand. Together, they create a powerful one-two punch that dramatically enhances both mitochondrial quality and quantity, leaving you with cellular energy systems that are far more robust than either therapy could achieve on its own.
Hydrogen Gas Therapy works like having a gentle, protective bodyguard for your mitochondria while simultaneously activating multiple cellular cleanup crews. Unlike other therapies that work through a single pathway, hydrogen gas is remarkably versatile, operating through several different mechanisms at once to create what researchers describe as the ideal environment for mitochondrial health.
One of hydrogen’s key talents is activating a specialized cleanup coordinator called FUNDC1—remember, this is one of those molecular disposal coordinators we discussed earlier that directly connects damaged mitochondria to the cellular recycling system. Think of hydrogen as providing FUNDC1 with a significant productivity boost, enabling it to identify and tag problematic mitochondria more efficiently.
At the same time, hydrogen gas fine-tunes your cellular energy sensor system, known as AMPK signaling. AMPK acts like your cell’s fuel gauge and efficiency monitor, constantly assessing energy levels and deciding when to activate cleanup processes. Hydrogen helps calibrate this system for optimal performance, ensuring that mitophagy kicks in at precisely the correct times and intensities.
What makes hydrogen therapy particularly elegant is its dual protective action. While it’s busy activating your cellular cleanup crews to remove damaged mitochondria, it’s simultaneously acting as a selective antioxidant, neutralizing only the most harmful free radicals while allowing beneficial oxidative signals to function normally. It’s like having a security system that can distinguish between dangerous intruders and helpful maintenance workers, protecting your mitochondria from oxidative damage while allowing everyday cellular communication to continue uninterrupted.
This creates what scientists call an ‘optimal environment’ for mitochondrial health—a perfect balance where old, damaged components are efficiently removed while healthy mitochondria are protected and preserved.
Intravenous Compound Therapies
NAD+ Supplementation represents the most direct approach to enhancing mitophagy through sirtuin activation. Clinical studies have demonstrated that NAD+ precursors, such as nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), can restore mitophagy function in aged cells and extend healthspan in animal models. The therapy works by providing the essential cofactor needed for sirtuin function, thereby restoring the cellular machinery responsible for maintaining mitochondrial quality. The best form of therapy is IV NAD.
Spermidine Therapy has emerged as one of the most potent mitophagy-enhancing compounds available. Research indicates that spermidine activates mitophagy through the PINK1/Parkin pathway, thereby extending lifespan across multiple species. The compound works by inhibiting histone acetyltransferases and promoting the expression of genes related to autophagy. Clinical applications of IV spermidine therapy show promise for reversing age-related mitochondrial decline.
Resveratrol Administration activates SIRT1 and promotes mitophagy through multiple mechanisms. The compound enhances mitochondrial biogenesis while promoting the selective removal of damaged mitochondria, thereby creating a comprehensive approach to maintaining mitochondrial health. Resveratrol’s effects on mitophagy appear to be particularly pronounced in combination with other longevity-promoting interventions.
Epigallocatechin Gallate (EGCG) modulates autophagy pathways and supports mitochondrial function through its antioxidant properties. While EGCG may suppress certain aspects of mitophagy under specific conditions, its overall effect appears to support mitochondrial health by reducing oxidative damage and promoting cellular resilience.
Curcumin Therapy enhances autophagy through the SIRT1/AMPK/mTOR pathway, promoting the clearance of damaged cellular components, including mitochondria. The compound’s anti-inflammatory properties complement its autophagy-enhancing effects, creating a comprehensive approach to maintaining cellular health.
Coenzyme Q10 (CoQ10) Supplementation supports mitochondrial function and provides substrate for the electron transport chain while offering antioxidant protection. While CoQ10 may not directly activate mitophagy pathways, it supports overall mitochondrial health and reduces the burden of oxidative damage that necessitates the activation of mitophagy.
Quercetin Administration provides senolytic activity that complements mitophagy by removing senescent cells that contribute to tissue aging. The compound’s antioxidant properties also support mitochondrial function and may indirectly enhance mitophagy by reducing cellular stress.
Urolithin A represents one of nature’s most powerful mitophagy activators, working like a master conductor that orchestrates your cellular cleanup symphony. It is taken orally. This remarkable compound is produced by beneficial bacteria in your gut when you consume pomegranates, walnuts, and certain berries, but here’s the catch—not everyone can make it effectively due to differences in gut microbiome health. What makes urolithin A so special is its ability to activate multiple mitophagy pathways simultaneously: it cranks up your cellular energy sensor (AMPK) to signal the need for cleanup, turns down the mTOR pathway that usually blocks cleanup activities, and directly boosts production of the PINK1 and Parkin proteins we discussed earlier. These molecular quality inspectors tag damaged mitochondria for removal. Studies show that urolithin A not only removes cellular garbage but also enhances the formation of autophagosomes (specialized disposal bags) and helps recycle their components into functional building blocks for new, healthy mitochondria. For patients seeking to optimize their cellular health, urolithin A represents one of the most scientifically validated natural approaches to enhancing the mitophagy process that becomes increasingly important as we age.
Therapeutic Synergies: Combining Modalities for Optimal Results
The most promising clinical approach to enhancing mitophagy involves the strategic combination of environmental and pharmacological interventions. For example, combining NAD+ therapy with intermittent hypoxia can provide both the molecular substrate (NAD+) and the cellular stimulus (hypoxia) needed for optimal sirtuin activation and mitophagy enhancement.
Similarly, combining cold therapy with compounds like spermidine or resveratrol may create synergistic effects that exceed the benefits of either intervention alone. These combinations target multiple pathways simultaneously, providing comprehensive support for mitochondrial health and cellular resilience. The bottom line is that Pur-Form can “stack” different modalities together to increase mitophagy and increase your healthspan.
– Dr. P