I recently shared a review of a new scientific study on LinkedIn that prompted deeper reflection on peripheral neuropathy, a condition I see far too often in clinical practice. Peripheral neuropathy is becoming increasingly common, especially among people with diabetes and those who have undergone chemotherapy. The central question raised by this research was both simple and profound. If many neuropathy cases are driven by mitochondrial dysfunction, can improving mitochondrial health actually reverse nerve damage?
Based on emerging science, the answer may be yes.
A Breakthrough Discovery in Neuropathy Research
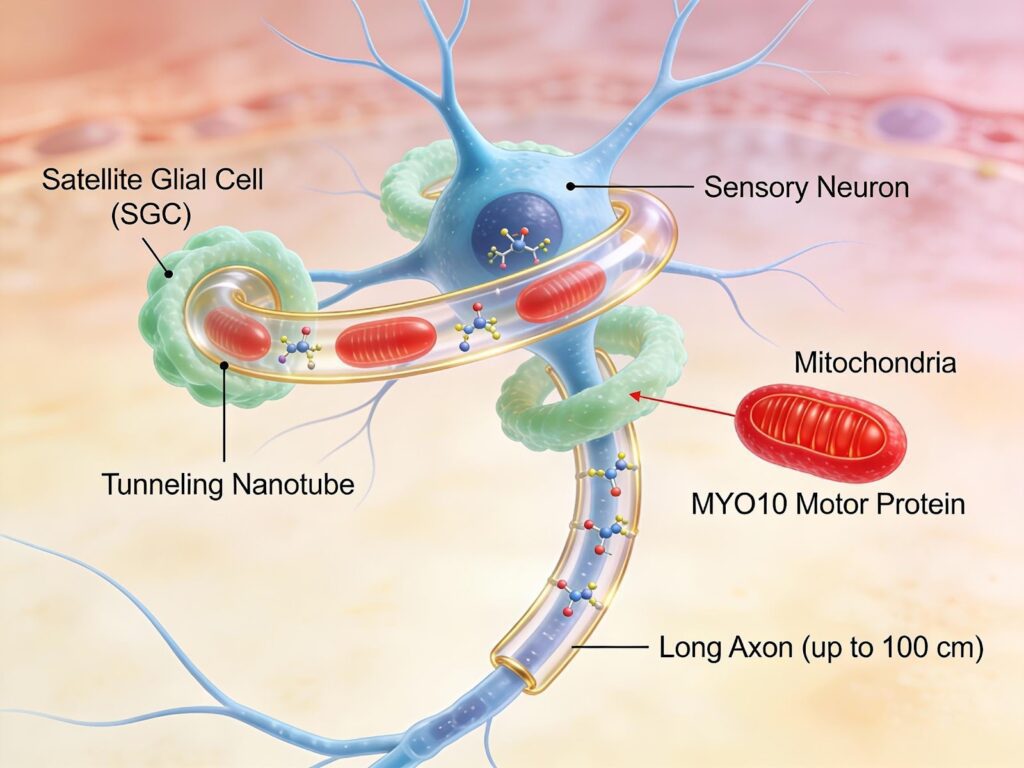
In January 2026, a groundbreaking study published in Nature revealed an entirely new mechanism behind peripheral neuropathy. Researchers discovered that specialized support cells called satellite glial cells play a critical role in keeping sensory nerves healthy. These cells surround sensory neurons in structures called dorsal root ganglia, which sit alongside the spinal cord and act as relay stations for all sensation traveling from the body to the brain.
What the study revealed is remarkable. Satellite glial cells actively donate healthy mitochondria to nearby sensory neurons. Mitochondria are the energy generators inside cells, and this transfer happens through tiny cellular bridges known as tunneling nanotubes. These nanotubes physically connect glial cells to neurons, allowing energy producing mitochondria to be shared when neurons are under stress.
When this mitochondrial support system works properly, nerves remain healthy. When it fails, nerve degeneration and chronic pain develop. Most importantly, the study showed that restoring healthy mitochondria can reverse nerve damage.
This finding reframes peripheral neuropathy as a disease of failed cellular support rather than irreversible nerve death.
Why Nerves Have Such High Energy Needs
Sensory nerves face a unique challenge. Some nerve fibers extend nearly one meter from the spinal cord to the feet. Maintaining these long structures requires enormous amounts of energy. Neurons cannot meet this demand on their own.
This is where satellite glial cells step in. They act as energy suppliers, constantly transferring fresh mitochondria to neurons based on activity level. The more active the nerve, the more mitochondrial support it receives.
The researchers were able to visualize this process using advanced imaging techniques and confirmed that the transfer increases when nerves are under stress. This system is not optional. When scientists intentionally blocked mitochondrial transfer in healthy animals, nerve degeneration and pain developed within days.
What Goes Wrong in Diabetes and Chemotherapy
In diabetes and after chemotherapy, this protective system breaks down.
In diabetic patients, satellite glial cells produce fewer tunneling nanotubes and express lower levels of the motor proteins required to move mitochondria. The physical connection between glial cells and neurons weakens, and mitochondrial transfer slows dramatically.
Chemotherapy causes a different but equally damaging problem. It directly injures the mitochondria inside glial cells. Even when transfer occurs, the mitochondria being donated are dysfunctional and cannot support nerve health.
The result is a cascade of damage including oxidative stress, nerve hyperactivity, axonal degeneration, loss of small nerve fibers in the skin, and persistent neuropathic pain.
Reversing Neuropathy by Restoring Mitochondria
The most encouraging finding from this study is that nerve damage was reversible.
When researchers injected healthy human satellite glial cells into diabetic mice, nerve fiber density improved and pain decreased. This benefit only occurred when the glial cells had intact mitochondria and functional transfer machinery.
Even more striking, injecting isolated healthy mitochondria alone was enough to restore nerve function. This confirms that mitochondrial health is not just supportive but central to nerve recovery.
Translating Science Into a Clinical Strategy
This research supports a new therapeutic framework for peripheral neuropathy. Instead of focusing only on nerve symptoms, treatment should aim to restore mitochondrial production, quality control, transfer efficiency, and nerve regeneration.
Below is a clinically grounded approach based on current evidence.
Supporting Mitochondrial Production With NAD Precursors
NAD is essential for mitochondrial energy production and repair. IV NAD, IV Niagen, or oral nicotinamide riboside help restore mitochondrial function and stimulate the creation of new mitochondria. Studies show these therapies improve nerve conduction, preserve small nerve fibers, and reduce neuropathic symptoms in diabetic neuropathy.
By increasing the supply of healthy mitochondria, satellite glial cells are better equipped to support stressed neurons.
Improving Mitochondrial Quality With Urolithin A
Urolithin A activates mitophagy, the process that removes damaged mitochondria. It also stimulates the creation of new, healthy mitochondria. In neuropathic pain models, urolithin A reduces pain, lowers inflammation, and promotes nerve regeneration.
This ensures that glial cells donate only high quality mitochondria rather than dysfunctional ones.
Protecting Mitochondria With Targeted Peptides
Several mitochondrial peptides show promise in neuropathy.
SS 31 stabilizes mitochondrial membranes, improves energy production, and reduces oxidative stress. MOTS c activates cellular energy sensing pathways and reduces inflammation without the side effects seen with traditional pain medications. Humanin analogs protect neurons from stress and stimulate mitochondrial growth.
These peptides support mitochondrial resilience in both glial cells and neurons.
Regenerating Nerves With Repair Peptides
Peptides such as BPC 157 and TB 500 promote nerve growth, blood vessel formation, and tissue repair. GHK Cu plays a particularly important role by stimulating nerve growth factors, increasing mitochondrial production, reducing oxidative stress, and supporting anti inflammatory pathways.
Together, these peptides create an environment where repaired nerves can survive and function.
Supporting Mitochondrial Function With Alpha Lipoic Acid
Alpha lipoic acid improves mitochondrial efficiency, reduces oxidative damage, and has demonstrated clinical benefit in neuropathic pain. It also improves blood flow to nerves, supporting the energy demands of regeneration.
A New Paradigm for Peripheral Neuropathy
This research fundamentally changes how we should think about peripheral neuropathy. It is not simply a disease of dying nerves. It is a disease of failed cellular support.
By restoring mitochondrial health in satellite glial cells and improving their ability to transfer energy to neurons, we may be able to reverse nerve damage rather than merely manage symptoms.
For clinicians, this represents a shift toward treating the root cause. For patients, it offers genuine hope.
Peripheral neuropathy does not have to be a one way decline. With the right biological support, nerves can heal.
Dr. P