Let us do a quick comparison between a ketogenic diet and a regular diet which depends on glucose. Glucose is the primary energy source for almost every cell in the body. This is because it can be broken down into energy much more quickly than any other fuel source, and it does this without the help of the mitochondria (the main energy producing component of the cell). Using glucose for fuel, however, comes with some negative effects. What we gain in quickness, we lose in efficiency. During the process of sugar burning more free radicals also called Reactive Oxygen Species (harmful compounds that can cause cell damage these are referred to as ROS) are released and less energy is created than when we use ketones and fat for fuel. The ROS harm cells in many different ways and advance aging on many different levels. On the other hand, Ketones are a more efficient fuel source that inhibits the production of free radicals and reactive oxygen species. This leads to a host of benefits, especially for the brain cells that use ketones instead of sugar for fuel when glucose levels are low. For example, studies done on people with different types of cognitive issues from Parkinson’s disease to epilepsy confirm that using ketones as fuel can improve brain function tremendously. However, the benefits of burning ketones for energy doesn’t stop in the brain. Many other cells like muscle cells also benefit from the use of ketones (more on that later), but you can’t reap these benefits unless you use up your sugar reserves first.
I have done a survey of some of the articles on the Ketogenic diet and most of them are parroting the same information. Most of these sites are missing some salient points about the Keto diet. They are not aware of the implications that the ketone bodies have on aging pathways which have a profound effect on our health landscape.
The ketogenic diet tries to bring carbohydrates down to less than 5 percent of a person’s daily caloric intake – which means eliminating most grains, fruit, starchy vegetables, legumes and sweets. Instead, it replaces those calories with fat. One myth that needs to be mentioned is that proteins are contra-indicated in a keto diet. Typically, proteins are not a problem. In some circles there is a misconception. Many low carb, high fat advocates believe excess protein can turn into sugar in your bloodstream through a process called gluconeogenesis and knock down your ketone levels. There is no evidence that consuming excess protein will increase glucose production from gluconeogenesis. Gluconeogenesis (GNG) is a metabolic pathway that allows your liver and kidneys to make glucose from non-carbohydrate sources. To clarify, you don’t need to eat any high carb foods to survive, but make no mistake — your body needs glucose and glycogen to keep you healthy (even on ketosis) and it will get this via survival mechanisms like gluconeogenesis.
There are a handful of cells in your body that can only use glucose to survive, including red blood cells, kidney medulla (inner part of the kidney), testicles and some parts of your brain. Ketones can cover up to 70% of your brain’s energy needs while glucose from GNG covers the rest. The other organs can’t metabolize ketones at all, so gluconeogenesis provides them with enough glucose to remain healthy.
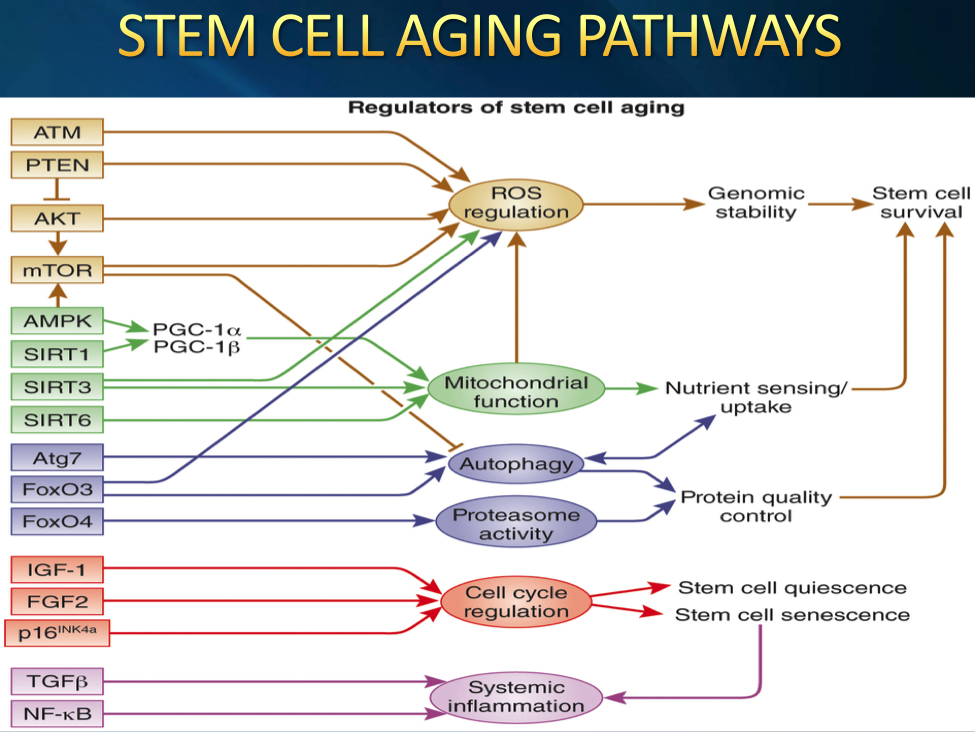
The Ketogenic Diet seems to have a direct effect on many of these aging pathways. Let us take a look at how the Ketogenic Diet may affect the various pathways. It is well known that caloric restriction extends lifespan. No generally accepted theory has been proposed to explain these observations. However, we now realize that the life span extension produced by caloric restriction can be duplicated by the metabolic changes induced by ketosis. Ketone bodies protect neurons against multiple types of neuronal injury and the underlying mechanisms are similar to those of calorie restriction and of the ketogenic diet. The following diagram gives an idea of some of the duties of ketone bodies.
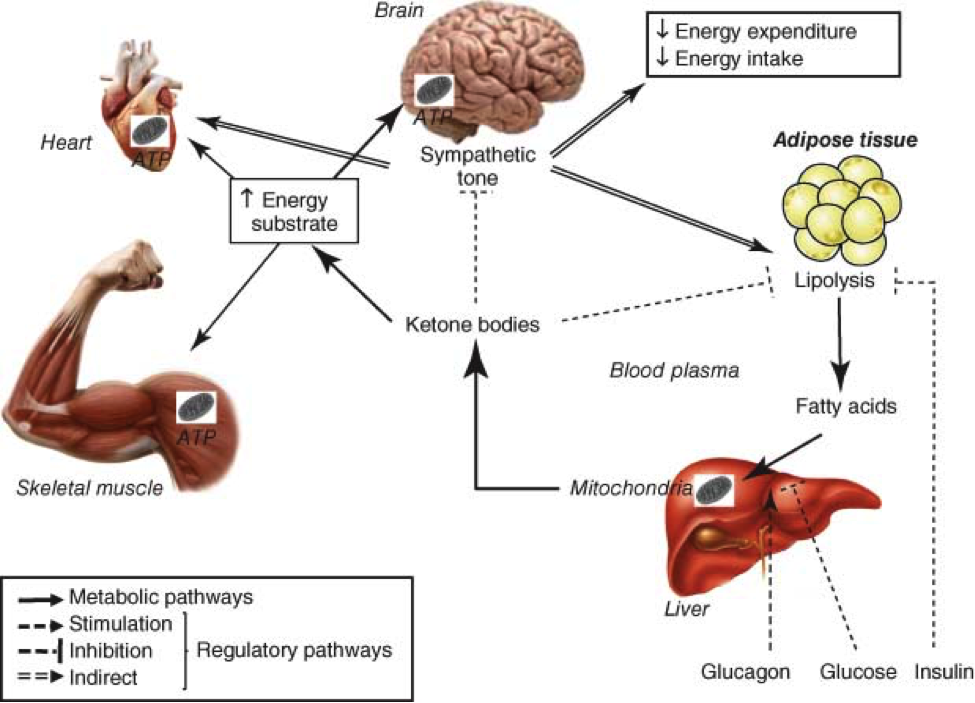
The diet’s high fat, low carbohydrate composition reduces glucose utilization and promotes the production of ketone bodies. Ketone bodies are a more efficient energy source than glucose and improve mitochondrial function and biogenesis, increased health span and lifespan and cellular energy production. When we are improving mitochondrial function, we are stimulating the Sirtuin genes which can be found on the first diagram as Sirt1,3, and 6. These same Sirtuin genes are the same ones that are stimulated by vigorous exercise, certain supplements such as Resveratrol, Pterostilbene, NAD, and finally calorie restriction. Thus, we can see the ketone bodies can dramatically affect the Sirtuin genes to help up regulate the production of ATP by stimulating the mitochondria. In many anti-aging circles ATP stimulation is considered one of the holy grails. Ketone bodies as fuel source are more efficient to burn into energy: ketone bodies require only one molecule of NAD+ per molecule of Co-enzyme A, whereas glucose needs 4 molecules of NAD+. As we can see ketone bodies as fuel allow us to have more NAD available. Co-enzyme A is important component in the Krebs cycle. The Krebs cycle is where ATP is made. The more NAD+ available the more ATP that can be produced.
But Ketone bodies go beyond being used as a fuel source. They themselves perform signaling activities in a way similar to growth factors. Intriguingly, the ketone body (which is also called BHB) might also be a metabolic intermediary of the benefits of calorie restriction and fasting. Long viewed as a simple carrier of energy from the liver to peripheral tissues during prolonged fasting or exercise, βOHB or Ketone bodies also possesses signaling activities. It therefore joins a small but growing list of metabolic intermediaries that affect gene expression via modifications of the DNA. These changes on the DNA ultimately affect the production of messenger RNA. Messenger RNA than turns on certain genes by giving them commands to produce certain growth factors etc. The following diagram gives us an idea of some of the various effects of ketone bodies in our bodies. Many of the effects we see in the following diagram are the result of Ketone body signaling.
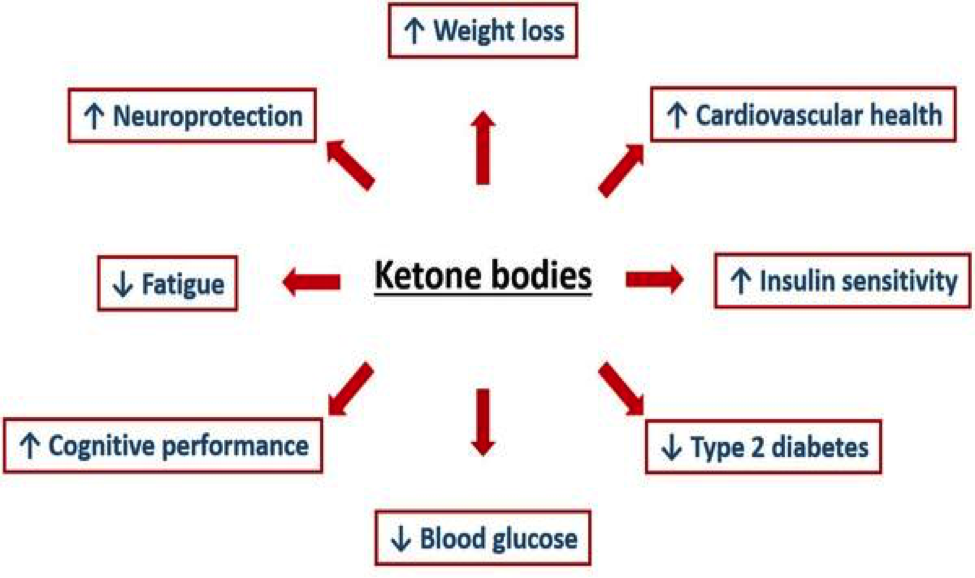
We can see that the effects of Ketone bodies are wide ranging. These ketone bodies and their intermediaries may be key links between variations in the cellular environment and the epigenetic changes associated with increased health span and lifespan. Epigenetics, as a simplified definition, is the study of biological mechanisms that will switch genes on and off. Epigenetics affects how genes are read by cells, and subsequently whether the cells should produce certain proteins.
Environmental factors such as nutrition dramatically alters cellular metabolism and many also alter the epigenetic regulation of gene expression. Ketones will increase the metabolic coenzyme nicotinamide adenine dinucleotide (NAD), a marker for mitochondrial and cellular health. Furthermore, NAD activates downstream signaling pathways (such as the sirtuin enzymes) associated with major benefits such as longevity and reduced inflammation; thus, increasing NAD is a coveted therapeutic endpoint. The literature is now ablaze with information on NAD and earlier this year Time Magazine presented an article calling NAD based supplements a possible true “Anti-aging Pill”. This assumption is not very far off the mark. Based on differential NAD+ utilization during glucose vs. ketone body during energy generation, it appears that a Ketogenic Diet will increase the NAD+/NADH ratio. The more NAD+ available the more ATP that can be produced. ATP production is thought to be a key factor in health and anti-aging.
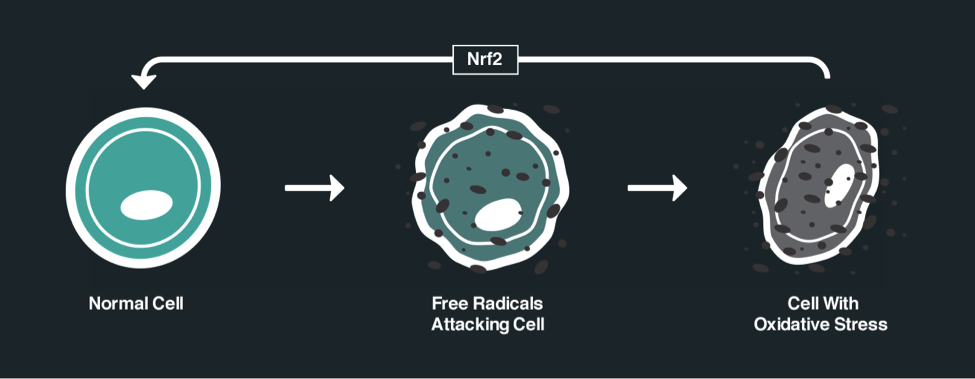
What else do ketone bodies stimulate? Another important pathway in the body is called the NRF2 pathway. The NRF2 (nuclear factor erythroid-derived 2-related factor 2) pathway is the cellular antioxidant system. The Nrf2 pathway has been referred to as the master regulator of antioxidant, detoxification and cell defense gene expression. We can think of Nrf2 pathway as a “thermostat” within our cells that senses the level of oxidative stress and other stressors and turns on internal protective mechanisms. Nrf2 is a potent modulator of antioxidant response and can rapidly target oxidative stressors. Ketone bodies increase mitochondrial glutathione levels by activating the Nrf2 pathway, thus reducing oxidative stress. It modulates the ratio between the oxidized and reduced forms of nicotinamide adenine dinucleotide (NAD+/NADH) which ultimately raises the ATP production. The following diagram shows the NrF2 pathway. We can see the difference between a normal cell and a cell that has suffered from oxidative stress.
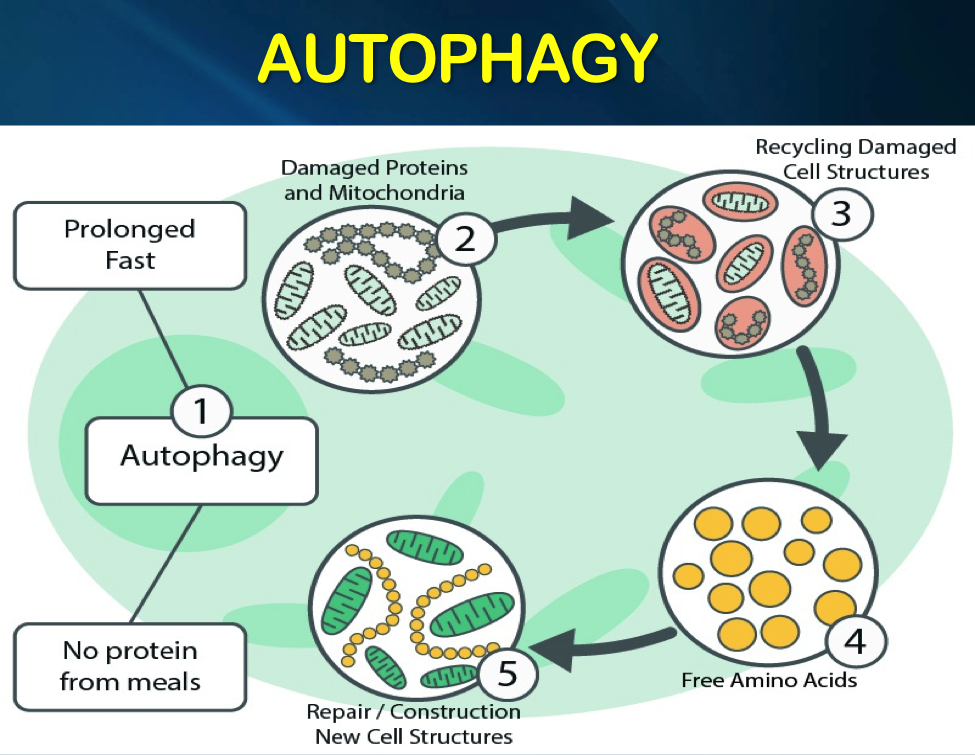
One characteristic of aging is the accumulation of damage, and this is largely due to the failure of autophagy to attain normal functional levels. It can be seen that bringing levels of autophagy to youthful levels can ameliorate aging by clearing out damaged parts of the cell. Cells use autophagy to get rid of damaged proteins and organelles, to counteract the negative effects of ageing on the body. Most anti-aging treatments and protocols have something in common: they all cause an increase in autophagy. Autophagy is central to extending lifespan and to avoiding the diseases of aging.
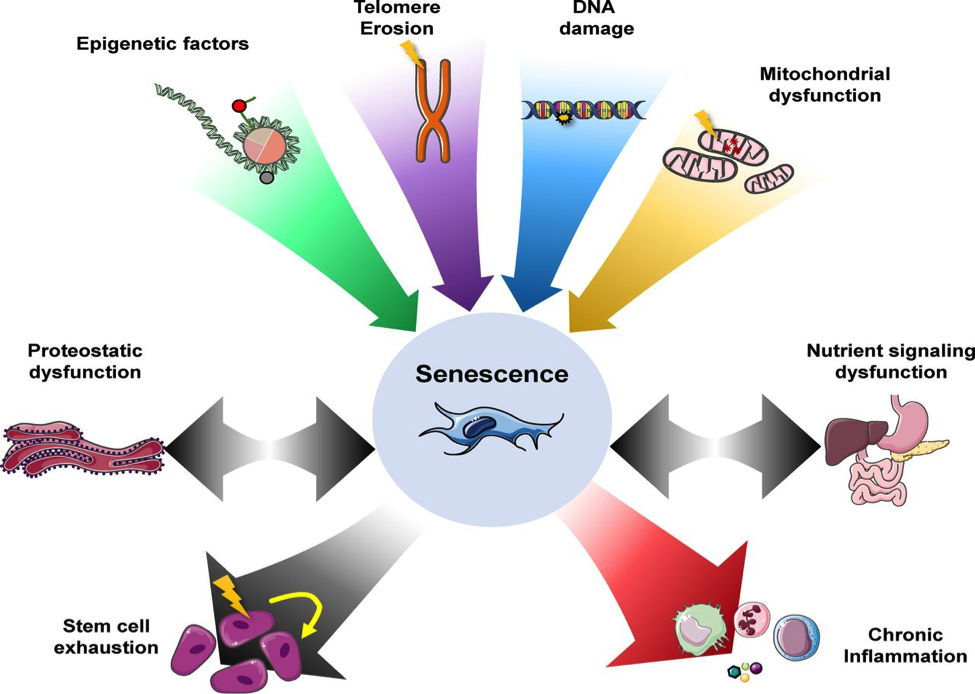
Senescent cells will accumulate growth factors, proteases, and inflammatory factors that disrupt normal tissue function. Senescent cells have long been implicated in aging and decreased success in stem cell procedures. By contrast, cellular quiescence is a reversible state of arrested growth; quiescent cells do not divide, but under the right conditions, they can re-enter the cell cycle and divide again. This state usually occurs when nutrition or growth factors are lacking, and it is thought to be a way that cells can avoid entering a senescent state. The cellular quiescence mechanism also helps cells to preserve stemness and resist stress to their genes. Senescent cells can no longer multiple and divide. The researchers found that ketone bodies can promote cell division and prevent cells from becoming senescent.
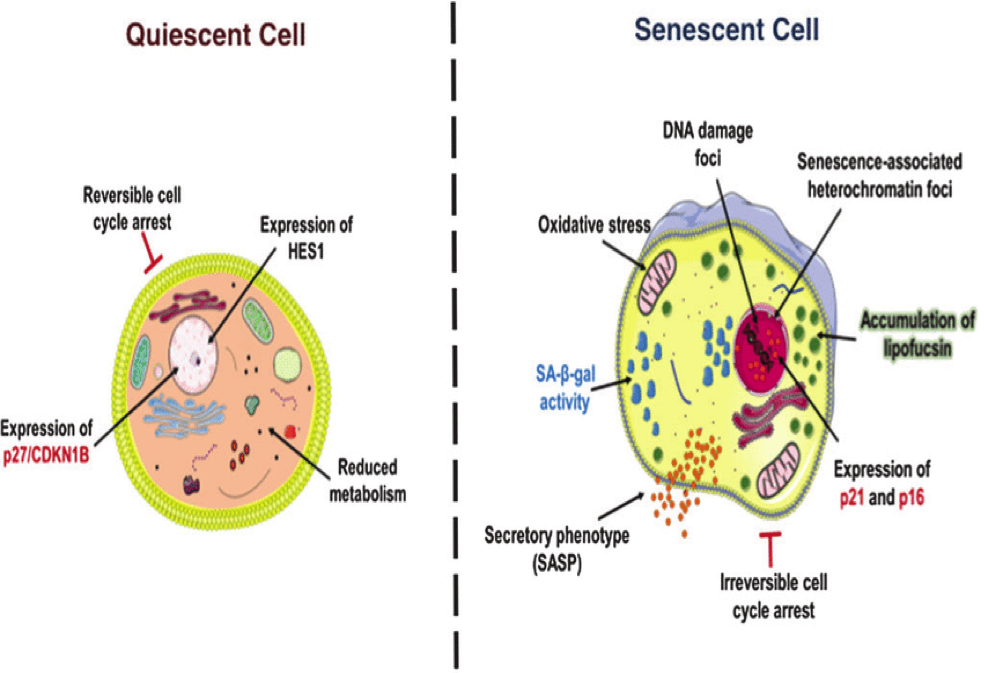
Although the above diagram is somewhat technical, it shows the difference between a quiescent cell and a senescent cell. We see that the senescent cell has a good deal of damage which has passed the point of no return. We now understand that a ketone body which is produced during calorie restriction or thru the Ketogenic diet will have anti-aging properties. In addition, the researchers found when the ketone body ( BBH also called β-Hydroxybutyrate) binds to a certain RNA-binding protein, this increases activity of a stem cell factor called Octamer-binding transcriptional factor (Oct4) in vascular smooth muscle and endothelial cells (Endothelium refers to cells that line the interior surface of blood vessels and lymphatic vessels, forming an interface between circulating blood or lymph in the lumen and the rest of the vessel wall). Oct4 increases a key factor against DNA damage-induced senescence. It is thought that vascular aging is one of the root causes of whole-body aging. Another interesting fact is that there seems to be a distinct relationship between ketone bodies and a certain protein that stimulates what is called the P-53 gene. The P-53 gene is also called the tumor suppressor gene. It attacks senescent cells and causes their demise. At the same time, it can cause repair of some damaged cells making them younger.
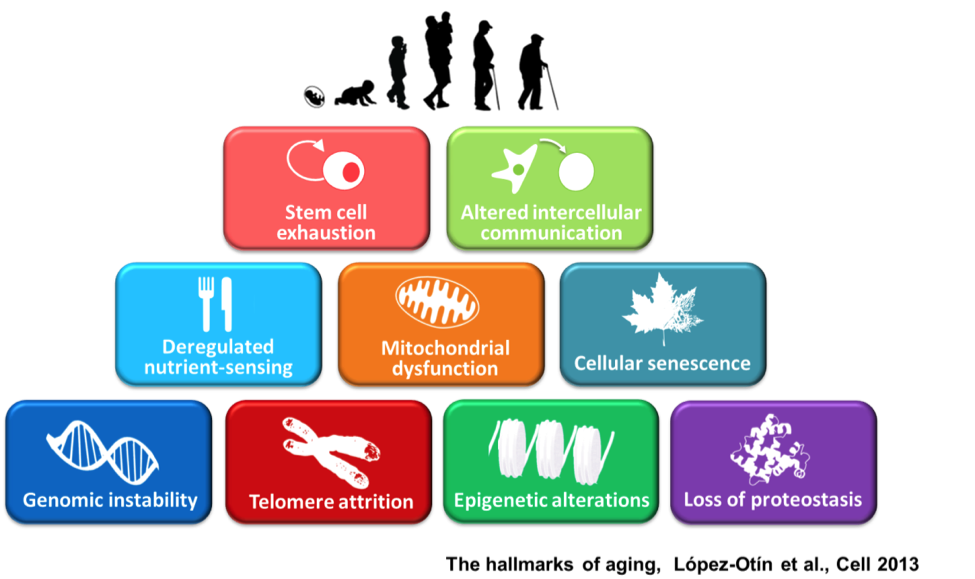
We must remember that success in stem cell procedures is dependent on successful manipulation of the Stem Cell Aging Pathways. I would like to impart one final thought. We need to use some common sense about this diet and “cheat” once in a while. Like I said when it comes to the ketogenic diet we need to use common sense but sometimes common sense is not very common. However, an occasional slice of pizza is not that bad.
Dr. P