I am giving a lecture in a few weeks on MUSE cells, and as I prepared, I was reminded why they continue to fascinate me. MUSE cells are, in many ways, the “supermen” of stem cells. They are powerful, adaptable, and remarkably clean in their behavior, meaning they carry little of the risk or instability seen in other stem cell types.
While reviewing the literature, I came across several papers connecting MUSE cells to two topics I have long been passionate about: V cells, also known as VSELs, and Intermittent Hypoxia Therapy. What stood out immediately was how closely these concepts are related.
MUSE cells and VSELs can be thought of as first cousins. They share a common lineage and many important characteristics, yet they behave differently and serve slightly different roles in the body. Intermittent Hypoxia Therapy turns out to be a powerful stimulator of both.
This connection helps explain why intermittent hypoxia has become one of my favorite tools in regenerative and longevity medicine.
What Is Intermittent Hypoxia Therapy
Intermittent Hypoxia Therapy, or IHT, is a safe and controlled treatment in which a person breathes air with reduced oxygen for short periods, alternating with normal or oxygen enriched air. Normal air contains about 21 percent oxygen. During IHT, oxygen levels are typically reduced to between 9 and 15 percent for brief intervals.
A simple way to think about it is altitude training at sea level. The body experiences the same beneficial stress it would feel at high altitude, but in a controlled medical setting.
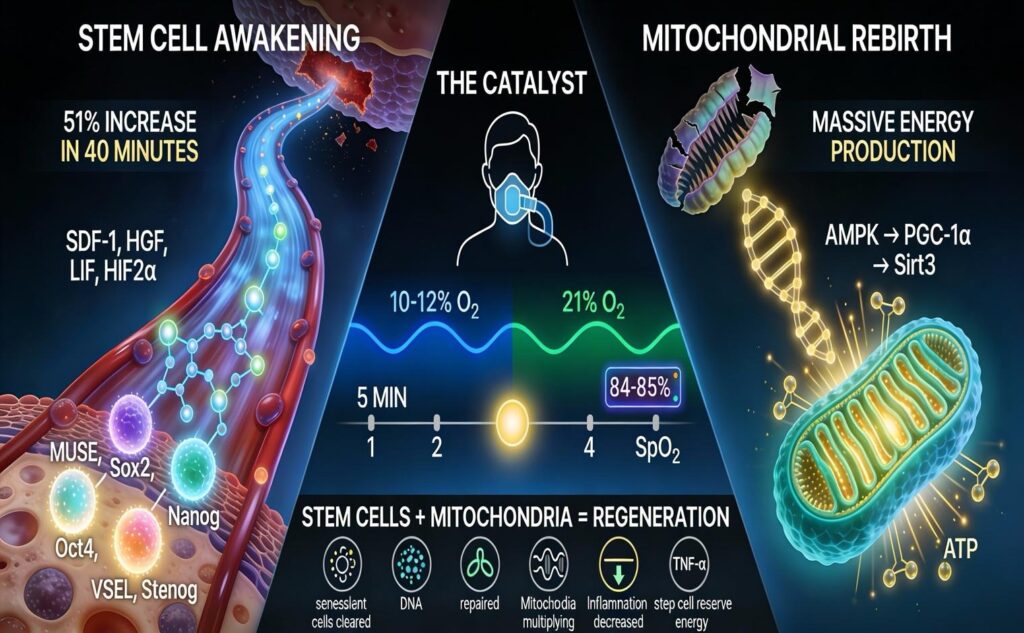
A typical session lasts about 40 minutes. During that time, the patient cycles between low oxygen breathing and normal or enriched oxygen breathing. While this may sound simple, it triggers two very powerful biological responses at the same time. First, it mobilizes highly regenerative stem cells from the bone marrow. Second, it stimulates the creation of new, healthy mitochondria, which are the energy producers inside our cells.
Mobilizing the Body’s Stem Cell Repair System
Deep within the bone marrow are rare stem cells that most people never hear about, yet they play a critical role in repair and regeneration.
MUSE cells, short for multilineage differentiating stress enduring cells, are pluripotent stem cells that exist naturally in adult bone marrow. They share many of the same regenerative markers as embryonic stem cells but without the ethical or safety concerns. These cells can become brain cells, heart cells, cartilage, skin, liver tissue, and more. They are extraordinarily versatile.
VSELs, or very small embryonic like stem cells, are also pluripotent and naturally present in adult bone marrow. They express master control genes such as Oct4, Sox2, and Nanog, which give them tremendous regenerative potential.
Under normal conditions, both MUSE cells and VSELs remain largely dormant. Intermittent hypoxia acts as a wake up call. It signals the bone marrow to release these cells into the bloodstream, where they can travel to areas of damage, inflammation, or degeneration.
The Science Behind Stem Cell Mobilization
Human studies have shown just how quickly this process occurs. In a landmark study, healthy adults underwent a single 40 minute session of intermittent hypoxia. Circulating stem cells increased by over 50 percent after only the second hypoxic cycle. Within 30 minutes after treatment, these cells began leaving the bloodstream and migrating into tissues that needed repair.
After two weeks of daily sessions, blood stem cell counts actually dropped. This was not a negative finding. It indicated that the stem cells had successfully exited circulation and entered tissues to begin healing.
Animal studies show similar results. Mice exposed to intermittent hypoxia mobilized pluripotent VSELs from bone marrow into circulation, activating over 1,100 genes related to brain development, blood vessel formation, and organ repair.
We also see this naturally in disease states. Stroke patients, for example, mobilize VSELs at nearly three times normal levels. These cells home directly to injured brain tissue. Intermittent hypoxia allows us to activate this same healing response without waiting for injury to occur.
How Hypoxia Signals Stem Cells to Move
When oxygen levels drop, the body releases a series of chemical messengers that act as guidance signals for stem cells. These include factors such as SDF 1, which attracts stem cells into circulation, HGF, which promotes cell movement and repair, and LIF, which helps maintain stem cell youth and regenerative capacity.
One of the most important players is HIF 2 alpha, a master regulator activated during hypoxia. This factor increases the proportion of MUSE cells and activates genes related to pluripotency. It is especially important for stimulating both MUSE cells and VSELs.
Together, these signals create a gradient that pulls stem cells out of the bone marrow and guides them precisely to where they are needed.
The Second Major Benefit: Mitochondrial Renewal
Mitochondria are the power plants of our cells. They produce ATP, the energy currency that allows every organ and system in the body to function. Each cell contains hundreds or even thousands of mitochondria, and their health directly influences metabolism, inflammation, brain function, and aging.
As we age, or when we experience chronic stress or inactivity, mitochondria become damaged and inefficient. This leads to fatigue, brain fog, muscle weakness, metabolic dysfunction, and accelerated aging.
The solution is mitochondrial biogenesis, the process of removing damaged mitochondria and creating new, healthy ones.
How Intermittent Hypoxia Builds New Mitochondria
Brief cycles of low oxygen followed by reoxygenation send a powerful signal to cells that more energy capacity is needed. This activates a well documented genetic pathway.
First, AMPK, the cell’s energy sensor, is activated. AMPK then turns on PGC 1 alpha, the master regulator of mitochondrial creation. Once activated, this pathway instructs cells to build new mitochondria. Sirt3 is also activated, improving mitochondrial efficiency and resilience.
The result is the production of brand new mitochondria with intact membranes, organized internal structures, and optimal energy output.
Electron microscope studies confirm this visually. After several weeks of intermittent hypoxia therapy, mitochondria appear more numerous, healthier in shape, and far more efficient at producing ATP. This is not a theory. It is observable cellular change.
What This Means Clinically
These cellular improvements translate into real world benefits. In neurological recovery, intermittent hypoxia has been shown to support brain mitochondrial regeneration after stroke, improving motor function and recovery.
Athletes using intermittent hypoxia alongside training demonstrate greater gains in mitochondrial mass and energy efficiency compared to training alone.
Importantly, these benefits are long lasting. Improvements in mitochondrial function persist for months after treatment ends, indicating true cellular remodeling rather than a temporary boost.
Why Stem Cells and Mitochondria Work Best Together
Stem cell repair requires enormous amounts of energy. MUSE cells and VSELs must remove damaged cells, differentiate into new tissue, secrete growth factors, and rebuild structure. All of this depends on ATP.
Intermittent hypoxia creates both the workforce and the energy supply. Stem cells are mobilized, and mitochondria are renewed. Together, they address many of the fundamental drivers of aging, including inflammation, stem cell exhaustion, mitochondrial dysfunction, and cellular senescence.
The Clinical Protocol
In a medical setting, intermittent hypoxia is delivered using specialized equipment that precisely controls oxygen levels. Sessions typically involve oxygen levels between 9 and 12 percent, with continuous monitoring to maintain safe oxygen saturation.
Treatments last about 40 minutes and are often performed daily for two weeks, followed by a maintenance phase several times per week. Patients are comfortable during sessions and can relax, read, or work.
Extensive clinical research supports the safety of this therapy, with no serious adverse events reported in human studies.
Who May Benefit
Intermittent hypoxia therapy may benefit individuals with neurological conditions, cardiovascular and metabolic disease, musculoskeletal injuries, athletic performance goals, and those focused on healthy aging and longevity.
It can also be used strategically alongside MUSE cell infusions. In this setting, intermittent hypoxia prepares tissues, reduces inflammation, enhances energy production, and improves stem cell retention and function.
Final Thoughts
Intermittent Hypoxia Therapy remains one of the most powerful and elegant tools we use. It mobilizes the body’s own regenerative stem cells, restores cellular energy production, dramatically reduces inflammation, and addresses multiple hallmarks of aging at once.
Other methods of increasing stem cells exist, such as pharmaceutical stimulation, but they are costly, carry risks, and do nothing to improve mitochondrial health. As I often say, exercise remains one of the best medicines we have, and intermittent hypoxia builds on many of the same biological principles.
Whether used alone or alongside advanced regenerative therapies, intermittent hypoxia offers a scientifically validated way to unlock the body’s innate capacity for repair, energy, and longevity.
Dr. Purita